Growing Together Fall 2019
pH and Water Quality Influence on Pesticide Spray Solution

Water is the primary carrier of agricultural chemicals. Water has the job of delivering pesticides to the plant, fungal spores or insect to achieve the goal of protecting crops. Water quality is determined by chemical properties such as pH, which is the measure of acidity and alkalinity, and the level of organic minerals present such as water hardness determined by level of minerals such as calcium, magnesium, and potassium. Pesticides can be sensitive to water chemistry and quality.
Some are affected by high pH levels and once the pH reaches above 7, certain pesticides can undergo alkaline hydrolysis. Alkaline hydrolysis breaks down the parent pesticide molecule into charged or ionic molecules, also known as cations and anions. In this form, a pesticide’s ability to perform can be altered. Uptake through the plant leaf, inability to bind at its target site, and in the ionic form, binding with hard water minerals or charged soil sediment that may occur in spray water, are a few critical events that can be influenced by alkaline hydrolysis.
Benefits of maintaining optimum pH of pesticide molecule:
Uptake
A whole or non-ionic chemical is also considered lipophilic or fat loving. A leaf cuticle has a large waxy portion that is also lipophilic. This lipophilic pesticide is more soluble in the waxy cuticle tissue and therefore moves more rapidly into the leaf. For pesticides, whose mode of action is to interfere with a plant biochemical process or be made available to sap sucking insects, rapid movement into the leaf and plant cells is critical.
Effect of Water Quality on Pesticide Availability
When the pesticide is in a charged or ionic state, it is capable of binding with mineral cations such as calcium, magnesium or iron that are present in hard water spray solutions. Once bound to a cation mineral in hard water, that molecule can no longer easily move through the leaf cuticle or bind to its target site of action.
Spray water pulled from a ditch may contain sediments of soil that include organic matter or clay. These sediment particles contain negatively charged sites that can bind to positively charged, cation pesticide molecules. In addition to influencing your overall spray operation, for example plugged nozzles, the pesticide bound to the charged soil or organic matter sediment is no longer free to easily move into the leaf and bind to the target site
Degradation
Hydrolysis is considered the beginning of a pesticide’s breakdown or degradation. At a certain pH level, a formulated product can remain stable for a period of time after which hydrolysis will begin. The published half-life of a pesticide can provides the optimum pH for the solution and how long it can last at that level. The half-life of a pesticide is defined as the length of time required for 50% of the pesticides active ingredient to breakdown.
In general, insecticides are more sensitive to high pH and alkaline hydrolysis than fungicides and herbicides.
There are certain classes of pesticides that are also sensitive to acid hydrolysis (low pH). These are fewer than those sensitive to alkaline hydrolysis, but it is best to be aware of a chemicals behavior when subjected to either condition.
Key points to remember:
- Determine the pH of spray water prior to adding the pesticide. Check it several times a year.
- Test the hardness of water for the presence of minerals.
- Check the pesticide label or MSDS for optimum pH level or ask your PCA or manufacturer rep.
- Use a quality pH adjuster and buffering agent to bring pH to the necessary level before adding chemical (usually between 6.0 – 7.0).
- Use as clean of spray water as possible as soil sediments can bind some pesticides.
- Know the half-life of the chemical (check the MSDS, ask your PCA or manufacturer rep)
- The longer the chemical sits in solution the more opportunity to degrade. Try to mix up only what can be sprayed that day.
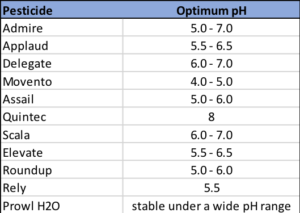
Examples of optimum pH level for some commonly used pesticides
pH Testing
Tools used to determine pH can run from a few dollars into hundreds of dollars. Battery powered digital pH meters provide the most accurate reading. A much cheaper tool is test/indicator papers that can be purchased at a swimming pool or aquarium supply store. They are not as reliable and can be as much as one to two pH points off. Remember pH is a logarithmic scale with each value 10 times greater or lesser than the next level so a misreading of one or two points may be critical (e.g. pH of 8 is ten times more alkaline than a pH of 7).
pH Adjusting Compounds
There are many products available that are used to adjust and buffer spray water pH. Ammonium sulfate used to condition hard water can drop spray water pH, but only slightly. Products designed to lower the pH and buffer water can be found at the local Grow West® location.
Hard Water Testing
Calcium and Magnesium minerals in water can, along with low pH, reduce the effectiveness of pesticides, especially some herbicides. If historical record of your water hardness is not available, take a sample to a local lab for analysis. There are some test/indicator papers that read Total Hardness but again, these are only a good general tool. Check with your PCA for available labs. Ammonium sulfate is the most common remedy for hard water. Once in solution with the hard water, ammonium sulfate will split into an ammonium cation and sulfate anion. The sulfate anion will tie up the Ca++ and Mg++ cations keeping them from binding with the available pesticides.
Web Sites for Further information
Effects of Water pH on the Stability of Pesticides – Michigan State University Extension
Effect of pH on Pesticide Stability and Efficacy – Pesticide Safety Education Program
Water Quality Affects Herbicide Efficacy – Oregon State Extension
